Which Best Expresses the First Law of Thermodynamics
The hotness or coldness of a body. The mathematical expression that show the first law of thermodynamics is as follows.

The First Law Of Thermodynamics Physics
The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods.

. Physics Exam Review 1. Temperature is measured in. The First Law of Thermodynamics.
The second law is about quality. The first law of thermodynamics is a version of the law of conservation of energy adapted for thermodynamic processes distinguishing three kinds of transfer of energy as heat as thermodynamic work and as energy associated with matter transfer and relating them to a function of a bodys state called internal energy. Changed from one form.
Thermal energy always flows naturally from a cold object to a hot object and never naturally from a hot object to a cold object. The amount of energy in the Universe is always constant. Q 0.
Work input is directly proportional to heat and the constant of proportionality is called. AP Physics Unit 8-9 Exam 1. Which statement represents the second law of thermodynamics.
First law of thermodynamics formula. What is the first law of Thermodynamics. Thermodynamics first law states that it is impossible to create or destroy energy.
According to this law the total amount of energy and matter remains constant in the Universe. It can be converted from one form to another. The second law states that this conservation of energy is from high-quality energy to low-quality energy.
We discussed that in The First Three Days of the Earth when we introduced the. Change in internal energy ΔU -100 J. Which process is represented by the PV diagramcurve.
The first law of thermodynamics is the law of the conservation of energy which states that although energy can change form it can be neither be created nor destroyed. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. In equation form the first law of thermodynamics is ΔU Q W.
The first law states the conservation of energy in which energy transforms into other forms of energy. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. Changed from one form.
Temperature is a quantity which expresses. The first law of thermodynamics expresses the quantity term of energy. Work done W 100 J.
There are some processes that can remove thermal energy from a source and convert it entirely into mechanical energy. -100 0 W. The law of conservation of energy states that.
The First Law of Thermodynamics is an expression of the principle of conservation of energy. They have zero tolerance for nonconforming processes. The first law of thermodynamics arguably the most important is an expression of the principle of conservation of energy.
Kelvin K Celsius C. The change of internal energy of a closed system will be equal to the heat addedrelease toby the system minus the work done to slash a buy a system. The laws of thermodynamics govern all physical processes without exception.
Which equation best expresses the first law of thermodynamics assuming Q is heat U is internal energy and W is work. The FLT defines internal energy as a state function and provides a formal statement of the conservation of energy. Since it is an adiabatic process the heat exchanged will be zero.
Consistent with this principle the first law expresses that energy can be transformed ie. State the first law of thermodynamics. The first law of thermodynamics states the law of conservation of energy i.
Heat energy can neither be created nor be destroyed. Consistent with this principle the first law expresses that energy can be transformed ie. The reason is that a heated gas can do work on mechanical turbines or pistons causing them to move.
ΔU Q W. In equation form the first law of thermodynamics is ΔU Q W. The first law is simply the law of the conservation of energy including the equivalent energy of matter.
The first law of thermodynamics arguably the most important is an expression of the principle of conservation of energy. It states that energy is always conserved it cannot be created or destroyed. What is an adiabatic process.
Where is the change in internal energy Q is the amount if heat added to the system W is the work done by the system.

The Second Law Of Thermodynamics Violates The Basic Definition Of Thermodynamics

Solved First Law Of Thermodynamics Sign Convention The Chegg Com

Solved First Law Of Thermodynamics Sign Convention The Chegg Com
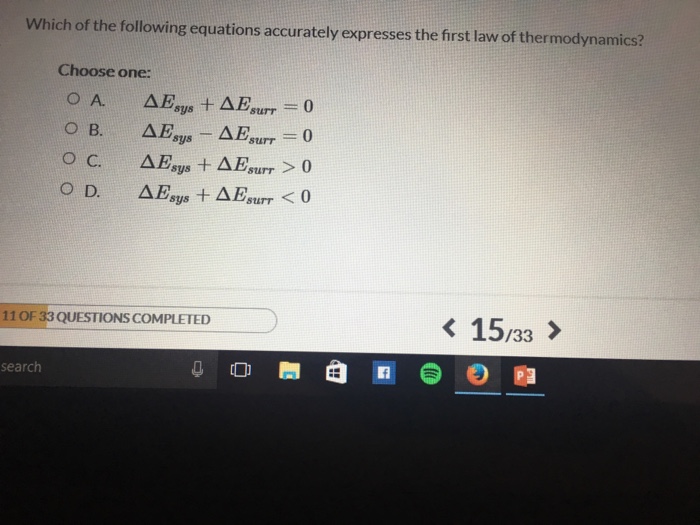
Solved Which Of The Following Equations Accurately Expresses Chegg Com

Learn About First Law Of Thermodynamics Internal Energy Chegg Com

What Is First Law Of Thermodynamics 9 Best Examples Equation

What Is First Law Of Thermodynamics 9 Best Examples Equation

What Is First Law Of Thermodynamics 9 Best Examples Equation

4 Laws Of Thermodynamics With Examples Very Simple
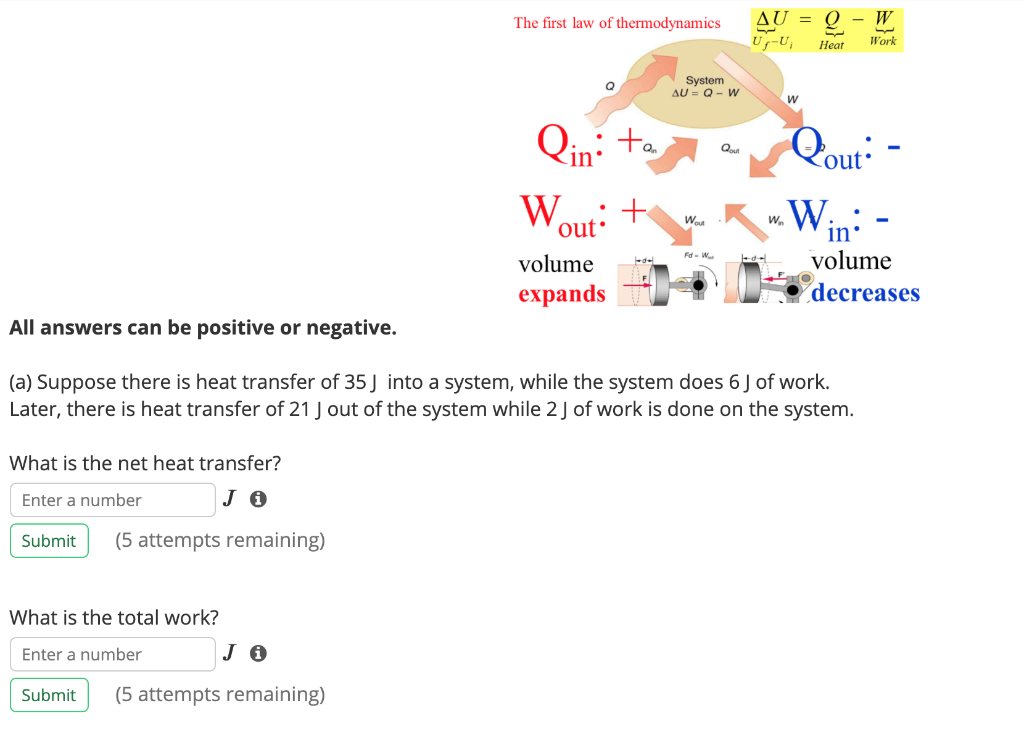
Solved First Law Of Thermodynamics Sign Convention The Chegg Com

First Law Of Thermodynamics Internal Energy Video Khan Academy

The First Law Of Thermodynamics Physics

It Is So Universal As To Have Been Discovered And Recognized Empirically As A General Scientific Law Th Second Law Of Thermodynamics Thermodynamics Scientific

What Is First Law Of Thermodynamics 9 Best Examples Equation
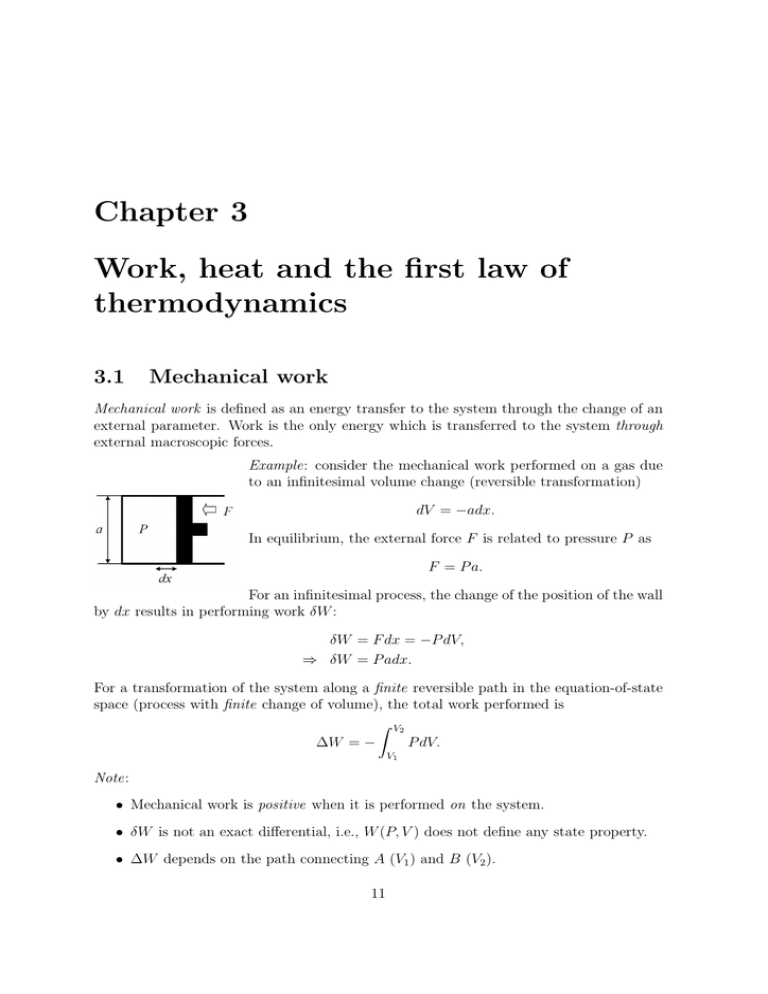
Chapter 3 Work Heat And The First Law Of Thermodynamics

What Is First Law Of Thermodynamics 9 Best Examples Equation

What Is The First Law Of Thermodynamics Article Khan Academy

4 Laws Of Thermodynamics With Examples Very Simple

What Is The First Law Of Thermodynamics Article Khan Academy
Comments
Post a Comment